(Original title: AFM: "Near-planarization" molecular design ideas improve the electrochemical performance of quinone-based organic electrode materials)
Recently, Dr. Yang Jixing and Professor Xu Yunhua of the School of Materials Science and Engineering of Tianjin University, have improved the electrochemical performance of quinone-based organic electrode materials based bn the "near planarization" molecular design idea. This improvement enables the optimized benzoquinone dimer molecule BBQB to have an initial specific capacity of 370 mAh/g. After 100 cycles at 0.1C, it can still maintain a specific capacity of 306 mAh/g (Figure 1c). This performance exceeds all the benzoquinone-based small molecule electrode materials and most of the polymer electrode materials reported so far.
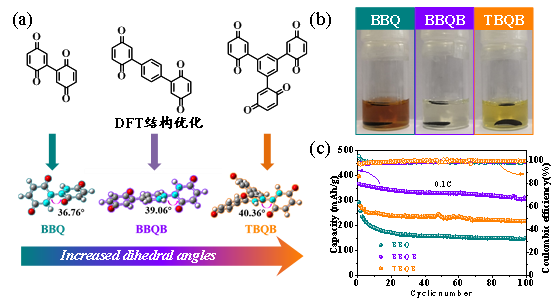
Figure 1. (a) Design the synthesis of three quinone-based compounds and their DFT-optimized molecular structure; (b) Comparison of the solubility of the electrode sheets of three quinone-based compounds in the same ether electrolyte; (c) Three The cycle performance of a quinone-based compound at 0.1C.
At present, the application scale of lithium ion batteries continues to expand. Inorganic electrode materials based on transition metal compounds are facing a series of challenges in terms of cost, energy density improvement and environmental friendliness. Compared with inorganic electrode materials, organic electrode materials have many advantages. . However, there are many practical challenges in the practical application of organic electrode materials. The most prominent problem is that the active molecules are easily dissolved in the organic electrolyte. The "loss" of active molecules will lead to a series of problems such as the measured specific capacity lower than the theoretical value, poor cycle stability. Benzoquinone is a high-value redox active unit with an average discharge voltage of 2.7 V and a theoretical specific capacity as high as 496 mAh/g. However, benzoquinone is easily soluble in organic electrolytes and own poor cycle stability. Optimizing the design of benzoquinone molecules to enhance the electrochemical performance is the current research focus of electrode materials.
This result was recently published on Advanced Functional Materials (https://onlinelibrary.wiley.com/doi/10.1002/adfm.201909597). The researchers extended this molecular design idea to the anthraquinone system, and greatly improved the electrochemical performance of anthraquinone-based electrode materials (ChemSusChem 2020, DOI: 10.1002/cssc.201903227).
